FDA Should Follow the 2021 FAO/WHO Expert Consultation & 1,576 Public Comments on Labeling Gluten
- Jon Bari

- Sep 2, 2022
- 23 min read
Updated: Feb 23, 2024
“We have been too quiet for too long. There comes a time when you have to say something.
You have to make a little noise. You have to move your feet. This is the time.”
-- Congressman John Lewis (1940-2020), The “Conscience of the Congress”
On April 19, 2022, the FDA published “Evaluating the Public Health Importance of Food Allergens Other Than the Major Food Allergens Listed in the Federal Food, Drug, and Cosmetic Act: Guidance for FDA Staff and Stakeholders – Draft Guidance”, April 19, 2022, Docket number: FDA-2021-N-0553 (“FDA’s Draft Guidance”).[1]
Based on the collective response and findings from scientists, physicians, patient advocacy groups, as well those with Celiac Disease and their loved ones and caretakers, the number of public comments submitted to the FDA indicates a clarion call to declare Gluten as a Major Food Allergen and require that Gluten be labeled on all packaged foods. In total, there were 1,903 comments submitted to the FDA, and of those, 1,576 comments mentioned Celiac and/or Gluten as a food allergen (82.8%). In the words of Congressman Lewis, we made a little noise.
We appreciate the opportunity to share our lived experience and provide our organizations' comments from Bari Consulting Group, Celiac Journey and @GlutenFreeFinds_pa on the FDA’s Draft Guidance (“Bari Comments”). The Bari Comments are intentionally very detailed in order to aggregate a lot of seemingly disparate, yet related, historical, legislative, regulatory, legal, medical and moreover patient advocacy perspective information. The Bari Comments are designed to ensure that the record is very clear since the FDA's Draft Guidance suggests that time has a way of blurring perspective for the FDA when it comes to the Food Allergen Labeling and Consumer Protection Act of 2004. Below is an edited version of our Executive Summary which provides a flavor of the more detailed Bari Comments.
Executive Summary
According to the FDA’s News Release on April 18, 2022, FDA Takes New Steps Regarding Evaluating Public Health Importance of Additional Food Allergens,
“Currently, the major food allergens [in the U.S.] are milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat and soybeans, though more than 160 foods are known to cause food allergic reactions. Sesame becomes the ninth major food allergen, effective Jan. 1, 2023.
‘The nine major food allergens don’t currently represent all foods nationwide that people are allergic to or that cause food hypersensitivities,’ said Susan Mayne, Ph.D., Director, Center for Food Safety and Applied Nutrition. ‘This draft guidance is part of the FDA’s efforts to evaluate emerging evidence about other non-listed food allergens that can cause serious reactions in a consistent and transparent manner, which can inform potential future actions to better help protect the health of consumers.’…
The draft guidance focuses on immunoglobulin E antibody (IgE)-mediated food allergies, which are capable of triggering anaphylaxis and are considered the most severe and immediately life-threatening food allergies.”
Therefore, with respect to the Celiac Disease community, which has been historically underserved and marginalized by our Federal government (i.e., funding medical research for Celiac Disease, labeling Gluten only with a voluntary scheme, disqualifying Celiacs from service in the military,[2] excluding Celiac Disease from the CDC’s Index of Diseases & Conditions, etc.), the FDA’s Draft Guidance presents an inequitable evaluation framework, as well as perpetuates flawed scientific, governmental and societal biases including:
A Gluten Free diet is all that is needed to treat Celiac Disease, as opposed to all that has ever been historically available to treat Celiac Disease.
With respect to labeling food products in the United States, the voluntary Gluten Free labeling scheme[3] sufficiently protects consumers who are on medically required and very restrictive Gluten Free diets, as opposed to all that has ever been historically utilized labeling-wise.
Othering the consumer protection needs for Celiacs in the United States with not evaluating the public health importance of Gluten as a food allergen because this Non-IgE-Mediated food allergy is not capable of triggering anaphylaxis and being immediately life-threatening, while seemingly ignoring that Celiacs face potentially life-threatening and severe adverse health effects that can arise through Gluten ingestion, including by way of example and not limitation: anemia, cancer, heart disease, immunological scarring, intestinal damage, malnutrition, etc.
Figure 1 - What is Food Allergy? The Similarities and Differences Between Non-IgE-Mediated Mechanisms with Celiac Disease & Typical IgE-Mediated Mechanisms

Figure 1 above illustrates the key near-peer similarities between food allergies that are Non-IgE-Mediated mechanisms with Celiac Disease (Gluten as a food allergen) and typical IgE-Mediated Mechanisms: potentially life-threatening, the only treatment is to strictly avoid the food allergen(s), and consumers’ reliance on food labels to know what is safe to eat.
Importantly, unlike food allergies with IgE-Mediated mechanisms, there is no rescue medicine (i.e., adrenaline or antihistamine) to treat the accidental ingestion of Gluten and the start of the auto-immune cascade in food allergy with Non-IgE-Mediated mechanisms such as Celiac Disease. Additionally, those with a Non-IgE-Mediated food allergy to Gluten cannot outgrow their food allergy – Celiac is lifelong (until such time as a cure may be developed).[4]
While U.S. consumers’ reactions to the top 9 major food allergens (Milk, Eggs, Fish, Crustacean Shellfish, Tree Nuts, Peanuts, Wheat, and Soybeans (plus Sesame as of January 1, 2023), collectively “Major Food Allergens”) and Gluten vary, their consumer habits are the same -- they avoid purchasing foods that contain the allergen(s) that cause a potentially life-threatening immunological adverse reaction. Notwithstanding the foregoing, the key difference from a consumer protection standpoint is that under the Food Allergen Labeling and Consumer Protection Act of 2004 (“FALCPA”), the labeling scheme for the top 9 Major Food Allergens in the U.S. is mandatory, but the labeling of Gluten is permissive.
Wheat is required to be labeled, but Gluten is not. Gluten is found in Wheat, Barley, Rye and most Oats.[5] Just because something is Wheat free does not mean its Gluten Free. In other words, whereas sufferers of the current top 9 Major Food Allergens in the U.S. rely on what ingredients are expressly included in required labeling disclosures of packaged foods, the Celiac community must rely only on what ingredients are excluded in voluntary Gluten Free labeling disclosures on packaged foods.
FDA's Draft Guidance is Inconsistent with Various Expert Committee Findings

The FDA’s Draft Guidance also suggests cognitive dissonance and confirmation bias by preemptively excluding Gluten containing grains from being considered in the context of evaluating the public health importance of food allergens other than the Major Food Allergens listed in the Federal Food, Drug, and Cosmetic Act (“FD&C Act”). This is because in part both the Food Allergen Labeling and Consumer Protection Act of 2004 and the FDA’s Draft Guidance are incongruent with the conclusions of international food safety authorities and expert committees comprised of scientists, regulators, physicians, clinicians and risk managers from academia, government and the food industry including:
Joint Food and Agriculture Organization of the United Nations ("FAO")/World Health Organization ("WHO") Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: fifty-third report of the Joint FAO/WHO Expert Committee on Food Additives. 2000. WHO Technical Report Series 896. World Health Organization, Geneva (“1999 FAO/WHO Expert Consultation”; also referred to as the “1999 Codex criteria” as detailed in the FDA’s Draft Guidance and cited as “FDA Ref. 25”).
Food and Agriculture Organization of the United Nations/World Health Organization. “Summary report of the Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens. Part 1: Review and validation of Codex priority allergen list through risk assessment.” 2021 (“2021 FAO/WHO Expert Consultation”; also referred to as “FDA Ref. 45” in FDA’s Draft Guidance). In 2022, the complete report was published, "FAO and WHO 2022 Risk Assessment of Food Allergens. Part 1 - Review and validation of Codex Alimentarius priority allergen list through risk assessment. Meeting Report. Food Safety and Quality Series No. 14, Rome."
The 1999 FAO/WHO Expert Consultation included Dr. Stephen Taylor, Professor and Founding Director (Retired) of the Food Allergy Research and Resource Program (“FARRP”), Department of Food Science and Technology at the University of Nebraska-Lincoln. Dr. Taylor served as one of six esteemed scientists on the Ad Hoc Panel on Food Allergens in February 1999 to provide advice to the Joint FAO/WHO Expert Committee on Food Additives about criteria for labelling food allergens. The 1999 FAO/WHO Expert Consultation determined:
“The revised list of those foods and ingredients known to cause food allergies and intolerance and whose presence should always be declared was identified as the following: cereals containing gluten (i.e. wheat, rye, barley, oats, spelt or their hybridized strains) and their products; Crustacea and products of these; Egg and egg products; Fish and fish products; Peanuts, soybeans, and products of these; Milk and milk products (lactose included); Tree nuts and nut products; and Sulfites in concentrations of 10 mg/kg or more.” (emphasis added)
The 2021 FAO/WHO Expert Consultation, which was an authoritative body chaired by the FDA’s Dr. Lauren Jackson, Chief, Process Engineering Branch, Division of Processing Science & Technology, Institute for Food Safety & Health, determined:
“Based on systematic and thorough assessments which used all three criteria (prevalence, severity and potency), the Committee recommended that the following should be listed as priority allergens: Cereals containing gluten (i.e., wheat and other Triticum species, rye and other Secale species, barley and other Hordeum species and their hybridized strains), crustacea, eggs, fish, milk, peanuts, sesame, specific tree nuts (almond, cashew, hazelnut, pecan, pistachio and walnut).”[6] (emphasis added)
According to the 2021 FAO/WHO Expert Consultation Meeting Report,
"The labelling of food allergens in pre-packaged foods plays a key role in protecting food allergic individuals, as no preventative clinical treatment is currently available. The list of major foods and ingredients known to cause hypersensitivity was included into the Codex General Standard for the Labelling of Packaged Foods (GSLPF) in 1999. There have been many scientific developments in the understanding of food allergens and their management since the original drafting of the GSLPF. Thus, in response to the request from Codex for scientific advice, including current evidence of consumer understanding of allergens, FAO and WHO convened a series of three expert meetings to provide scientific advice on this subject. The purpose of the first meeting of the Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens was to review and validate the Codex priority allergen list through risk assessment. This report focuses on the deliberations and conclusions of this meeting."
The 2021 FAO/WHO Expert Consultation also included several other expert scientists and physicians from the United States, including esteemed public servants from the FDA and the United States Department of Agriculture. See section in Bari Comments entitled, “2021 FAO/WHO Expert Consultation The Scientists Have Spoken”.
“The idea that you can get up here and talk about what you know – what the evidence, what the science is -- and know that’s it, let the science speak.” -- Dr. Anthony Fauci
The 2021 FAO/WHO Expert Consultation (FDA Ref. 45, Annex 1) was comprised of 20 (twenty) “experts” including:
Dr. Joseph Baumert, Professor and Director of the Food Allergy Research and Resource Program (FARRP), Department of Food Science and Technology at the University of Nebraska-Lincoln; (Pictured in top row of photograph, 3rd from left)
Dr. Lauren Jackson, Chief, Process Engineering Branch, Food and Drug Administration. Division of Processing Science & Technology, Institute for Food Safety & Health, who served as Chairperson of the Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens (FDA Ref. 45); (Pictured in top row of photograph, 1st person on left)
Dr. Stefano Luccioli, Medical Officer and Allergy Specialist at the Center for Food Safety and Applied Nutrition of the Food and Drug Administration, and Board-certified doctor in allergy/immunology who sees patients at the General Internal Medicine clinic at MedStar Georgetown University Hospital in Washington, D.C.; and Chairperson of Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens Part 3: Review and establish precautionary labelling in foods of the priority allergens (Pictured 2nd row of photograph, 4th person from left).
Dr. Stephen Taylor, Professor and Founding Director (Retired) of the Food Allergy Research and Resource Program (FARRP), Department of Food Science and Technology at the University of Nebraska-Lincoln. Dr. Taylor was also one of six scientists who served on the Ad Hoc Panel on Food Allergens met in Geneva, Switzerland in February 1999 to provide advice to the Joint FAO/WHO Expert Committee on Food Additives about criteria for labelling food allergens. Their treatise was memorialized in the 1999 FAO/WHO Expert Consultation; also referred to as the “1999 Codex criteria” as detailed in the FDA’s Draft Guidance and cited as “FDA Ref. 25”. (Pictured in bottom row of photograph, 2nd person from left).
In addition to these experts, the 2021 FAO/WHO Expert Consultation was comprised of 10 (ten) “resource persons” including two from the United States who work for the USDA and FDA:
Dr. J. Emilio Esteban, Chief Scientist of the U.S. Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS), who serves as the Codex Committee Chairperson from the United States; and
Dr. Douglas Balentine, Senior Science Advisor, Global Nutrition Policy Center for Food Safety and Applied Nutrition, FDA, who serves as the U.S. Delegate on Food Labeling on the Codex Committee.

It is ironic and troubling that while the FDA Ref. 25 and FDA Ref. 45 are cited as sources in the FDA’s Draft Guidance, the FDA’s Draft Guidance excludes salient findings in those treatises by the “scientists, regulators, physicians, clinicians, and risk managers from academia, government and the food industry”[7], and their conclusions to always declare (label) Gluten on food product labels in order to provide consumer protection for the Celiac community to whom ingesting Gluten is tantamount to eating poison and potentially life-threatening and life-debilitating.
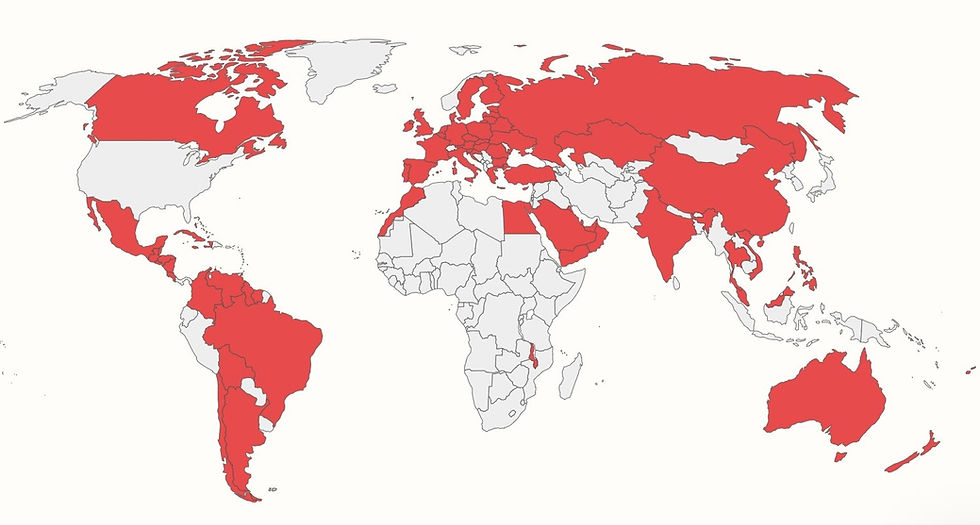
The global adherence to and implementation of FDA Ref. 25 and FDA Ref. 45 can be seen in how more than 85 countries worldwide require that Gluten be labeled on all packaged foods, according to the map and chart produced by the Food Allergy Research and Resource Program at the University of Nebraska-Lincoln.[8]
Figure 2 - Gluten Is Required to Be Labeled as a Major/Priority Food Allergen on Packaged Foods In More than 85 Countries Worldwide (shaded in red), Not Including the United States
Comments from Dr. Virginia Stallings
It is instructive to read Dr. Virginia Stallings comments to the FDA dated August 4, 2022. Dr. Stallings is board-certified nutrition pediatrician, Professor of Pediatrics and Director of the Nutrition Center at the Children’s Hospital of Philadelphia. In addition, Dr. Stallings served as the Editor and Chair of the National Academies of Sciences, Engineering, and Medicine, Committee on Food Allergies: “Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management and Public Policy.” This was published by the National Academies Press, 2016 and cited as FDA Ref. 2 in the FDA’s Draft Guidance.
The FDA appears to have been previously relying on FDA Ref. 2 to exclude the labeling of Gluten, and Dr. Stallings' August 4, 2022 comments rebut various FDA objections including that Celiac is not an allergy, that Gluten is not a food allergen and that Celiac is not potentially life threatening.
According to Dr. Stallings’ comments to the FDA,
“A gluten free diet is not all that is needed to treat Celiac Disease; rather a gluten free diet is all that has ever been historically available to treat Celiac Disease. Additionally, with respect to labeling food products in the United States, the voluntary gluten free labeling scheme does not sufficiently protect consumers who are on medically required and very restrictive gluten free diets. My strong recommendation is that gluten be labeled on all packaged foods in the United States, in accordance with the 2021 FAO/WHO Expert Consultation, just like it is in more than 85 countries around the world. I respectfully request that the FDA draft guidance be revised to include evaluating gluten as a food allergen and changing the voluntary labeling rule to a mandatory labeling rule to keep the 3 million Americans with Celiac Disease safe.”[9] (emphasis added)
In terms of consumer protection, the Celiac community would benefit greatly if the FDA and scientists would consistently refer to Celiac as a food allergy and Gluten as a food allergen. See section in Bari Comments entitled, “What’s In A Name? Celiac is a Food Allergy!”.
Additionally, it is important to note that a classification for Celiac Disease is not binary. In other words, if someone refers to Celiac as a digestive disease and/or an auto-immune disease, that is not mutually exclusive from Celiac also being a food allergy. A review of various written descriptions about Celiac Disease, and programs related thereto, from various Federal government agencies (i.e., FDA, National Institutes of Health (“NIH”), Centers for Disease Control (“CDC”)) suggests that this complexity has also enabled the ingrained othering by various government officials of a food allergy with Non-IgE-Mediated mechanism to be considered something else and something less serious than a typical food allergy, something else other than a digestive disease and/or something else other than an auto-immune disease.
Figure 3 - Celiac Disease Sits At The Intersection of
Food Allergies, Auto-immune Diseases and Digestive Diseases

While there are many similarities between food allergies with IgE-Mediated Mechanisms and Non-IgE-Mediated Mechanisms, including needing the same consumer protections with food labeling, it is also instructive to understand how Celiac Disease is uniquely complex since it sits at the intersection of food allergies, auto-immune diseases and digestive diseases. Figure 3 shows how Celiac can often be misunderstood. The complexity of this amalgam has seemingly made it more challenging for public servants, scientists and the Celiac community to address and protect the Celiac community’s needs. A review of various descriptions of Celiac Disease and programs related thereto from Federal government agencies suggests that this complexity has also enabled the othering by various government officials of a food allergy with Non-IgE-Mediated mechanism for Celiac to be considered something other than/less serious than a food allergy and Gluten something other than/less serious than a food allergen. See section in Bari Comments entitled, “What's In A Name? Celiac is a Food Allergy!”.
President Biden's Executive Order 13985 & The White House Conference on Hunger, Nutrition, and Health on September 28, 2022
In accordance with President Joe Biden’s Executive Order 13985, advancing equity requires a systematic approach to embedding fairness in the decision-making processes that all FDA functions are routed through. Using that lens and framework, the FDA’s Draft Guidance suggests systemic food privilege that contributes to food insecurity, and the FDA should work to redress historical inequities in various agencies’ policies and processes, including in the FDA’s Draft Guidance.
To that end, it is instructive to look at how the USDA defines food insecurity -- “Food insecurity is the limited or uncertain availability of nutritionally adequate and safe foods, or limited or uncertain ability to acquire acceptable foods in socially acceptable ways.” For those with food allergies such as Celiac Disease, we further define food insecurity as the limited or uncertain availability of food that is adequate, nutritious, safe and affordable, regardless of socioeconomic status, and limited or uncertain ability to acquire acceptable foods in socially acceptable ways.
These inequities serve as barriers to the equal protection under the law and fair evaluation of the public health importance of adverse reactions to food due to a food hypersensitivity that is mediated by immune mechanisms, including for Gluten as a potentially life-threatening and life-debilitating food allergy with a Non-IgE-Mediated mechanism. By way of example, independent and peer-reviewed analysis has shown that NIH funding of Celiac Disease research is not proportional to its disease prevalence or mortality in terms of a cohort of digestive diseases.[10] In addition to digestive diseases, further analysis has shown that NIH funded research of Celiac has also lagged behind in the biomedical imagination of other much more well-funded areas including autoimmune diseases, food allergies and foodborne illnesses. See section in Bari Comments entitled, “Disparities Among Gastrointestinal Disorders in Research Funding From the NIH”.
Alignment with the White House Conference on Hunger, Nutrition, and Health
As noted in Section II of the Bari Comments, "Preliminary Statement on Health Equity and President Biden’s Executive Order 13985", we believe that the FDA's work on evaluating food allergens aligns with President Joe Biden’s Executive Order 13985 & The White House Conference on Hunger, Nutrition, and Health in September. Specifically, we believe that Executive Order 13985 aligns with the White House Conference on Hunger, Nutrition, and Health ("White House Conference”) scheduled for September 2022, including with reducing diet-related disease,
“White House Conference “Pillar #2, Integrate nutrition and health: Prioritize the role of nutrition and food security in overall health, including disease prevention and management, and ensure that our health care system addresses the nutrition needs of all people.”
"If they don't give you a seat at the table, bring in a folding chair."-- Representative Shirley Chisholm (D-12, NY), the first African-American Congresswoman
Pernicious Impact of the Lack of Funding for Celiac Research
The FDA’s Draft Guidance suggests an admission of the pernicious impact that the lack of Federal research funding for Celiac has had with regard to how the FDA assesses and implements consumer protection. For example, the FDA’s Draft Guidance states,
“As discussed in section III.A, this document addresses the food allergies that have been most studied and understood clinically i.e., IgE-mediated food allergies. Therefore, the initial question for us to address when we evaluate the public health importance of a food or component of food as a food allergen is whether there is robust evidence that an adverse reaction to the food or component of food is IgE-mediated (Factor #1)” (emphasis added)
First, since Celiac research (Non-IgE-Mediated food allergy) has historically been underfunded, including from the NIH and Department of Defense (“DoD”), Celiac is not as very well studied (arguably one of the least studied food allergies) and understood clinically. Therefore, the FDA’s Draft Guidance suggests that Non-IgE-Mediated food allergies are not as important and do not deserve the same consumer protections with labeling as typical IgE-Mediated food allergies which are better funded and “have been the most studied and understood clinically.” The FDA’s Draft Guidance further suggests a classic negative feedback loop indicating how the Celiac community has been historically underserved by the Federal government, and moreover, how it will continue to be underserved based on being underserved in the past.
Moreover, the FDA's claim is inconsistent with the 2021 FAO/WHO Expert Consultation which concluded the following for both IgE-Mediated and Non-IgE-Mediated food allergies:
“Based on systematic and thorough assessments which used all three criteria (prevalence, severity and potency), the Committee recommended that the following should be listed as priority allergens: Cereals containing gluten (i.e., wheat and other Triticum species, rye and other Secale species, barley and other Hordeum species and their hybridized strains), crustacea, eggs, fish, milk, peanuts, sesame, specific tree nuts (almond, cashew, hazelnut, pecan, pistachio and walnut)." (emphasis added)
Unfortunately, in spite of our many precautions, our 9 year old son, Jax, has been “glutened” at times through accidental ingestion of Gluten and that has resulted in him experiencing GI issues commensurate with foodborne illness (food poisoning), and in addition, potential long-term complications including damage to his small intestine. Last Summer, Jax got violently ill when the chef at a restaurant thought a packaged food product was Gluten Free since it did not have Wheat labeled on the package, but the food product did have unlabeled Gluten in it. [11] That’s one real world example of getting Glutened and why we need Gluten to be labeled on packaged foods!
Food Insecurity Happens Every Day for Celiacs
As my 9 year old son Jax says, eating without fear is our hope. Food insecurity happens every day because of the constant threat of cross contact with Gluten, 80% of foods have Gluten in them, the limited availability of Gluten Free food (especially eating out of home), and Gluten is not required to be labeled on packaged foods in the US, like it is in 85 countries around the world including in Canada and across Europe.
In terms of being historically underserved by the Federal government, our research has informed our findings that the Celiac community in the United States has been left to fend for ourselves, literally and figurately. As a consequence, the Celiac community has received “half a loaf at twice the price” (see section in Bari Comments entitled, “The Economics of Celiac Disease The Financial Burden of the Gluten Free Diet” and Figure 9 herein for Market Shopping in the Philadelphia Area as of July 10, 2022). FALCPA’s voluntary labeling scheme has caused informational malnourishment and created a premium marketplace for Gluten Free food.
Until there is a cure for Celiac Disease, a treatment other than a strict Gluten Free diet for life, or even a rescue medicine that can be administered in the event of accidental ingestion of Gluten (i.e., epinephrine or antihistamine in the case of IgE-Mediated food allergy), the FDA can help save a life (or lives) or help someone (or some people) have a better life by protecting the Celiac community in an equitable and meaningful manner, and that includes starting with revising the FDA’s Draft Guidance and requiring that Gluten be labeled on all packaged foods.
The bottom line is that the FALCPA does not preclude the FDA from expanding via regulation the list of major allergens requiring identification under the FALCPA’s labeling scheme.[12] Section 203(b) states that the labeling requirements established under new section 403(w) “do not prevent the Secretary from requiring labels or labeling changes for other food allergens that are not major food allergens.”[13]
It Takes a Village - Team GF
We want to provide special thanks to Tricia Thompson and Gluten Free Watchdog for their tireless work to protect the Celiac community and leadership in responding to the FDA. It is a privilege to work with these engaged thought leaders and organizations in the Gluten Free community!
Some of the Major Comments Submitted to the FDA
Below are links to the some of the major comments regarding "Evaluating the Public Health Importance of Food Allergens Other Than the Major Food Allergens Listed in the Federal Food, Drug, and Cosmetic Act: Guidance for FDA Staff and Stakeholders – Draft Guidance”, April 19, 2022, Docket number: FDA-2021-N-0553:
Bari Consulting Group, Celiac Journey and Gluten Free Finds, "Sharing Our Lived Experience with Celiac Disease - Comments on 'Evaluating the Public Health Importance of Food Allergens Other Than the Major Food Allergens Listed in the Federal Food, Drug, and Cosmetic Act: Guidance for FDA Staff and Stakeholders -- Draft Guidance', August 16, 2022." FDA Comment ID: FDA-2021-N-0553-1584, FDA Tracking Number l6w-qe9m-4tjy, August 16, 2022. Cover Letter from Bari Consulting Group, Celiac Journey and Gluten Free Finds to Susan Mayne, Ph.D., Director, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration and Frank Yiannas, Deputy Commissioner for Food Policy and Response, U.S. Food and Drug Administration.
Beyond Celiac, FDA Comment ID: FDA-2021-N-0553-1353, FDA Tracking Number: l6q-v6ic-9kfi, August 12, 2022.
Brian Davis and Family, FDA Comment ID FDA-2021-N-0553-1385, FDA Tracking Number l6v-04b4-4gs6, August 15, 2022. Mr. Davis's son, Joshua Davis, was the special guest of President Joe Biden and First Lady Jill Biden at the 2022 State of the Union. Mr. Davis discussed how Gluten Free food is to Celiacs as to what Insulin is to Diabetics.
Celiac Community Foundation of Northern California, FDA Comment ID: FDA-2021-N-0553-1691, FDA Tracking Number: l6x-3son-9p1o, August 17, 2022
Gluten Free Watchdog, FDA Comment ID: FDA-2021-N-0553-0179, FDA Tracking Number l2u-t68j-qa01, May 9, 2022.
Dr. Amanda Muir, FDA Comment ID: FDA-2021-N-0553-1587, FDA Tracking Number l6w-qnl1-geu8, August 8, 2022. Dr. Muir is a Pediatric Gastroenterologist in the Division of Gastroenterology, Hepatology and Nutrition at the Children’s Hospital of Philadelphia. Dr. Muir is a physician-scientist with a laboratory studying mechanisms of inflammation in Non-IgE mediated food allergy (specifically eosinophilic gastrointestinal disorders), and she has a clinical practice dedicated to taking care of children with these disorders. According to Dr. Muir's FDA Comments,
"Eosinophilic esophagitis, or EoE, is a chronic allergic disease that affects pediatric patients and adults alike. In the setting of exposure to foods that are ubiquitous in the American diet, most commonly milk and gluten, an inflammatory process occurs in the esophagus leading to esophageal damage and narrowing. Celiac Disease is an autoimmune disorder in which exposure to gluten containing foods, causes, "face potentially life-threatening illnesses if they eat gluten, typically found in breads, cakes, cereals, pastas, and many other foods... There is no cure for celiac disease and the only way to manage the disease is to avoid eating gluten." Taken together, these two entities, Celiac Disease and EoE represent non-IgE mediated food allergies that would greatly benefit from specific gluten labeling."
National Celiac Association, FDA Comment ID FDA-2021-N-0553-0822, FDA Tracking Number: l4e-cf9x-ka7n, June 14, 2022.
Dr. Arunjot Singh, FDA Comment ID: FDA-2021-N-0553-1681, FDA Tracking Number: l6x-2tjb-tdx0, August 17, 2022. Dr. Singh is Assistant Professor of Clinical Pediatrics, Co-Director, Children's Hospital of Philadelphia's Center for Celiac Disease, Division of Gastroenterology, Hepatology & Nutrition, Perelman School of Medicine - University of Pennsylvania.
Dr. Virginia Stallings, FDA Comment ID: FDA-2021-N-0553-1169, FDA Tracking Number: l6g-mawc-nbs8, August 5, 2022. Dr. Stallings is board-certified nutrition pediatrician, Professor of Pediatrics and Director of the Nutrition Center at the Children’s Hospital of Philadelphia. In addition, Dr. Stallings served as the Editor and Chair of the National Academies of Sciences, Engineering, and Medicine, Committee on Food Allergies: “Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management and Public Policy.”
University of Chicago Celiac Disease Center, FDA Comment ID: FDA-2021-N-0553-1083, FDA Tracking Number l6d-sfof-noog, August 3, 2022.
Update
Call to Action: Submit Comments to FDA to Support Citizen Petition to Label Gluten as Major Food Allergen
Notes [1] Note that throughout the FDA Draft Guidance, there are citations to FDA “VIII. References” that are cited as “Ref. [X]” and those citations correspond to references in https://www.regulations.gov/document/FDA-2021-N-0553-0007 . Throughout these Comments, “FDA Ref. [‘X’]” refers to the documents cited in the FDA Draft Guidance.
[2] U.S. Department of Defense, DoD Instruction 6130.03, Medical Standards for Appointment, Enlistment, or Induction into the Military Services, Section 5.12.c.(3), May 6, 2018, https://www.esd.whs.mil/DD/
[3] The FDA’s final rule defining Gluten Fee for food labeling became effective on September 4, 2013, and August 5, 2014 was the date when FDA-regulated foods labeled Gluten Free must comply with all requirements established by the final rule. https://www.federalregister.gov/documents/2013/08/05/2013-18813/food-labeling-gluten-free-labeling-of-foods , and https://www.fda.gov/regulatory-information/search-fda-guidance-documents/small-entity-compliance-guide-gluten-free-labeling-foods#
[4] This diagram graphic is intended to convey that Non-IgE-Mediated food allergy with Celiac Disease and typical IgE-Mediated food allergies are both really dangerous and deserve equal treatment with respect to consumer protection with food labeling. The adverse health effects are listed in alphabetical order, and these health dangers are an illustrative, but not exhaustive list. This diagram will be discussed in greater detail herein, but it should be noted that this expands and updates information as was included in Table 2-1 in FDA’s Draft Guidance Ref. 2.
[5] Gluten containing grains are Wheat, Barley, Rye and most Oats. Wheat is already codified as a Major Food Allergen in the US. As such, the focus of these Comments is on adding Barley, Rye and Oats. While Oats are naturally Gluten Free, it is instructive to read the 1999 Codex Criteria (See FDA Ref. 25) which stated, “The revised list of those foods and ingredients known to cause food allergies and intolerance and whose presence should always be declared was identified as the following: Cereals containing gluten (i.e. wheat, rye, barley, oats, spelt or their hybridized strains) and their products.” Also see 2021 FAO/WHO Expert Consultation (See FDA Ref. 45). In addition, it is instructive to read the following from General Mills about oats being cross contaminated with Gluten, “Oats themselves do not contain gluten. However, oats cultivated in North America, Europe and even other parts of the world are commonly contaminated by gluten containing foreign grains, including wheat, barley, rye and triticale. This contamination is commonly known to come from various sources, mainly from the rotation of small grain crops on the same land, with residual contaminating seeds germinating with a seeded oat crop. In addition, contamination from other grains which are harvested, transported, stored and merchandized in common with oats is a contributing factor. As a result, it is not uncommon to find from 0.5% to 5.0% of these other grains mixed with commercially marketed oats. Therefore, absent dedicating land, harvesting equipment, transporting vehicles, storage units, packaging and production facilities, and the like only for use in connection with oats, cross contamination is inevitable.” Source: https://patents.google.com/patent/US9968937B2/en . It is also instructive to understand the market position and expertise of General Mills in the food industry. According to General Mills’ 2021 Annual Report, “We are a leading global manufacturer and marketer of branded consumer foods sold through retail stores. We also are a leading supplier of branded and unbranded food products to the North American foodservice and commercial baking industries. We are also a leading manufacturer and marketer in the wholesome natural pet food category. We manufacture our products in 13 countries and market them in more than 100 countries. In addition to our consolidated operations, we have 50 percent interests in two strategic joint ventures that manufacture and market food products sold in more than 120 countries worldwide.”
[6] “Due to the lack of data on prevalence, severity and/or potency, or due to regional consumption of some foods, the Committee recommended that some of the allergens, ... oats, ... should not be listed as global priority allergens but may be considered for inclusion on priority allergen lists in individual countries.”
[7] It is estimated that a large number of multinational companies in the food industry, including American firms which sell their food products abroad, already label Gluten on their products sold in countries outside of the US that require Gluten to be labeled on all packaged foods. In other words, the food industry has been helping to protect consumers for many years in labeling Gluten on their products which are sold in any one of the more than 85 countries around the world which require Gluten to labeled on all packaged foods, in accordance with the 1999 Codex Criteria and more recently with the 2021 FAO/WHO Expert Consultation.
[8] According to the University of Nebraska-Lincoln, the following countries require that Gluten be labeled on packaged foods: Anguilla, Antigua and Barbuda, Argentina, Australia, Austria, Bahamas, Barbados, Belarus, Belgium, Belize, Bermuda, Bolivia, Brazil, British Virgin Islands, Bulgaria, Canada, Cayman Island, Chile, China, Colombia, Costa Rica, Croatia, Cuba, Cyprus, Czech Republic, Denmark, Dominica, Egypt, El Salvador, Estonia, Fiji, Finland, France, Germany, Greece, Grenada, Guatemala, Guyana, Haiti, Honduras, Hong Kong, Hungary, India, Ireland, Italy, Jamaica, Kazakhstan, Kuwait, Latvia, Lithuania, Luxembourg, Malawi, Malaysia, Malta, Mexico, Montserrat, Morocco, Netherlands, New Zealand, Nicaragua, Oman, Philippines, Poland, Portugal, Qatar, Romania, Russia, Saint Lucia, Saudi Arabia, Singapore, Slovakia, Slovenia, Spain, St. Kitts and Nevis, St. Vin. and Grenada, Suriname, Sweden, Thailand, Trinidad and Tobago, Turkey, Turks and Caicos Island, Ukraine, United Arab Emirates, United Kingdom, Venezuela, Vietnam, and Yemen.
[9] FDA Comment ID: FDA-2021-N-0553-1169, FDA Tracking Number: l6g-mawc-nbs8
[10] “Disparities Among Gastrointestinal Disorders in Research Funding From the National Institutes of Health,” The American Gastroenterological Association, By: Emma Clerx, Harvard University; Sonia Kupfer, Celiac Disease Center at University of Chicago; and Daniel Leffler, North American Society for the Study of Celiac Disease, Beth Israel Deaconess Medical Center; September 4, 2017, https://www.gastrojournal.org/article/S0016-5085(17)36084-5/pdf
[11] See “Food Allergen Labeling And Consumer Protection Act of 2004 Questions and Answers”, Question 26, “What about food prepared in restaurants? How will I know that the food I ordered does not contain an ingredient to which I am allergic? FALCPA only applies to packaged FDA-regulated foods. However, FDA advises consumers who are allergic to particular foods to ask questions about ingredients and preparation when eating at restaurants or any place outside the consumer’s home.” https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-questions-and-answers#
[12] “See FALCPA 203(b), 21 U.S.C.A. 343(note); FALCPA 203(a), 21 U.S.C.A. 343(x). The Senate Committee Report states that it intends for any regulations issued by FDA requiring the identification of additional allergens to prescribe disclosure in “a manner consistent with” the FALCPA. S. Rep. No. 108-226, at 10.” “The legislation also adds a second misbranding provision to account for other food allergens. In particular, section 403(x) provides that FDA has the authority to require by regulation appropriate labeling of any spice, flavoring, coloring, or incidental additive ingredient that is, or includes as a constituent, a food allergen that is not a major food allergen. The committee does not intend the listing of all spices or flavorings in a product but intends that the Secretary will require the food allergen to be identified on the label in a manner consistent with this legislation.”
[13] H.R. Rep. No. 108-608, at 18. (2004).



